
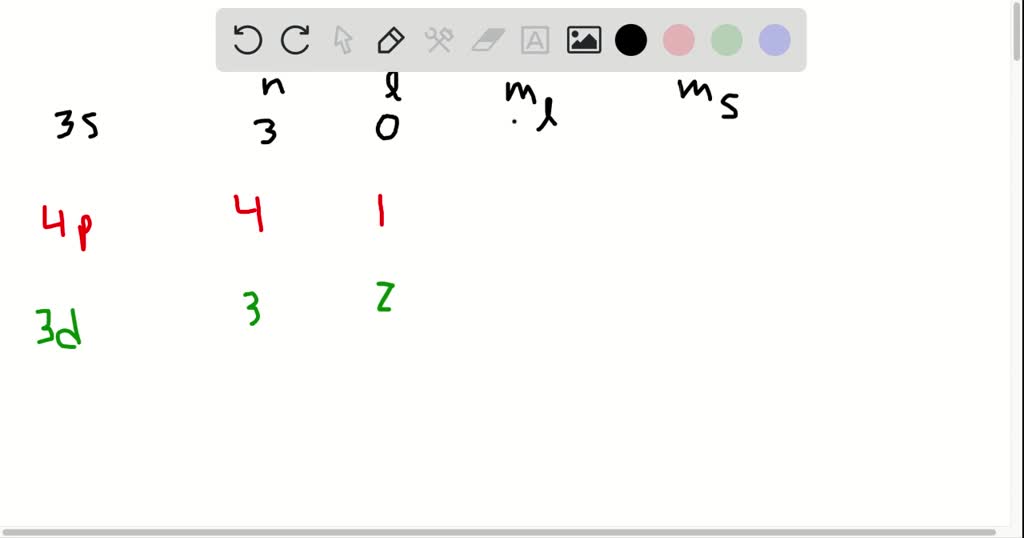
The next quantum number is the Magnetic Quantum Number, m l which shows the number of orbitals in the sublevel. Therefore, this combination represents the 3d sublevel:Įach d sublevel has five orbitals, and because each orbital can accommodate two electrons at most, the maximum number of electrons will be 10. For the sublevel (the type of the orbital), we need to look at the angular momentum quantum number, l, and when l = 2, we have d orbitals. Solution: The main energy level is given by the principal quantum number ( n), so this is an orbital in the 3 rd energy level. Draw the orbital diagram to explain your answer. Let’s put the orbitals corresponding to each value of l in a diagram as well:Įxample: Identify the main energy level, the sublevel, and the maximum number of electrons that can have the following quantum numbers: n = 3, l = 2. For example, for the second energy level, n = 2, and therefore, l = 0, 1, so it can have two values, and therefore, the second energy level has two sublevels – s ( l = 0) and p ( l = 1). So, how do we know what sublevels ( types of orbitals) a given energy level has? This is determined by the Angular Momentum Quantum Number ( l). For example, boron has two electrons in each s orbital of the first and second levels, and one electron in the p sublevel. We can see this in orbital diagrams where the orbitals are shown as boxes and electrons as arrows, we never put more than two arrows in the box. First, remember that each orbital, whether it is s, p, d, or f can accommodate two electrons at most. Now, a few important things about the orbitals and their electron capacity. So, for a given value of n:Į ( s orbital) < E ( p orbital) < E ( d orbital) < E ( f orbital) Notice again that within the same principal level, orbitals with a lower value of l have lower energy ( E) and therefore, are filled first. However, you should know, aside from the first energy level, all the others have sublevels, and these are the types of orbitals that we have talked about – s, p, d, and f. So, far we have talked about the main energy level. The number of types of orbitals matches the energy level: the first energy level has only 1 (s) orbital, the second has two types – s and p, the third has three – s, p, and d, and the fourth level has all four types of orbitals – s, p, d, and f. You can also look up more detailed images for the shapes and orientation of atomic orbitals in your textbook. S orbitals have a spherical shape, p orbitals are dumbbell-shaped, d orbitals are shaped like a cloverleaf, and f orbitals are characterized by more complex shapes. Each orbital has a characteristic shape shown below: Remember, there are four types of atomic orbitals – s, p, d, and f. There are orbits with fixed radii each associated with discrete energy, and this is described by the principal quantum number n. This is what we discussed about the Bohr model of the hydrogen atom. Remember, the energy level of the atom is given by the principal quantum number, n which can easily be determined based on the period (row) the atom is located in the periodical table. What orbitals a given atom has, and in which ones the electrons are located, depends on the energy level of the atom.

Let’s now discuss the quantum numbers one by one in more detail. So, one can visualize the information conveyed by quantum numbers getting more specific as we go from the principal quantum number to the spin quantum number: The Electron Spin Quantum Number ( m s) – shows the direction of the electron spin.The Magnetic Quantum Number, ( m l) – indicates the specific orbital within the energy sublevel.The Angular Momentum Quantum Number ( l) – indicates the energy sublevel, which is given by the type of the orbital ( s, p, d, f).The Principal Quantum Number ( n) – indicates the main energy level of orbitals and electrons.There are four quantum numbers that we are going to discuss:

Collectively, they all describe the electron configurations. Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron.


 0 kommentar(er)
0 kommentar(er)
